Clinical Applications
Rapid-myco technologies is developing a truly transformative diagnostic platform for the rapid detection of important and often life-threatening mycobacterial infections of humans and livestock.

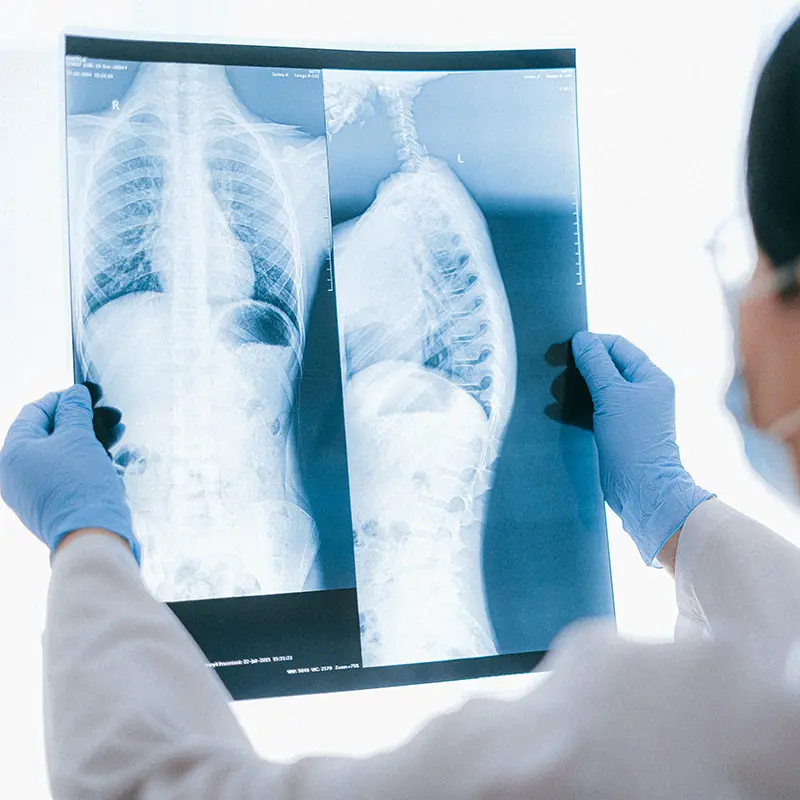
Human Tuberculosis (TB)
The treatment of TB, primarily caused by M. tuberculosis, if diagnosed early and accurately, is relatively straightforward and effective, requiring up to six months of appropriate antibiotic therapy. However, the accurate diagnosis of which Mycobacterium sp. is causing the infection is important because M. tuberculosis and M. bovis (zTB) infections requiring differing antibiotic regimes. Currently, differentiation of the two species after mycobacterial culture requires expensive and time-consuming spoligotyping which is often unavailable in developing countries.
RMT is seeking to transform TB diagnosis by developing duplex TB diagnostic assays on both PhMS-qPCR and LFT assay formats that will be capable of differentiating infections caused by M. bovis and TB.
RAPIDTB is a PhMS-duplex qPCR assay designed to detect and differentiate viable Mycobacterium bovis and Mycobacterium tuberculosis complex cells in the same patient sample. This PhMS-duplex qPCR assay will be applicable to original clinical specimens (blood or sputum), rather than a 6-8 week mycobacterial culture from these samples. A definitive diagnosis will be obtained within hours rather than weeks, enabling immediate administration of appropriate antibiotic treatment, which will be of great benefit to clinicians and patients.
As a complement to the PhMS-duplex qPCR assay for human TB, RMT is developing an antibody-based dual lateral flow test for detection and differentiation of zTB and TB patients, infected with M. bovis and M. tuberculosis complex (other than M. bovis), respectively. The test, RAPIDzTB, is specifically designed for use after mycobacterial culture (liquid or solid) to confirm presence of M. bovis or other M. tuberculosis complex species. The test takes ≤15 minutes and would obviate the need for expensive and time consuming spoligotyping.
RMT has received support from the World Health Organisation for the development of its suite of TB tests, which WHO has confirmed would greatly enhance its global TB detection and control programme.
Livestock Applications
Read more about the applications for Johne’s Disease and Bovine Tuberculosis.
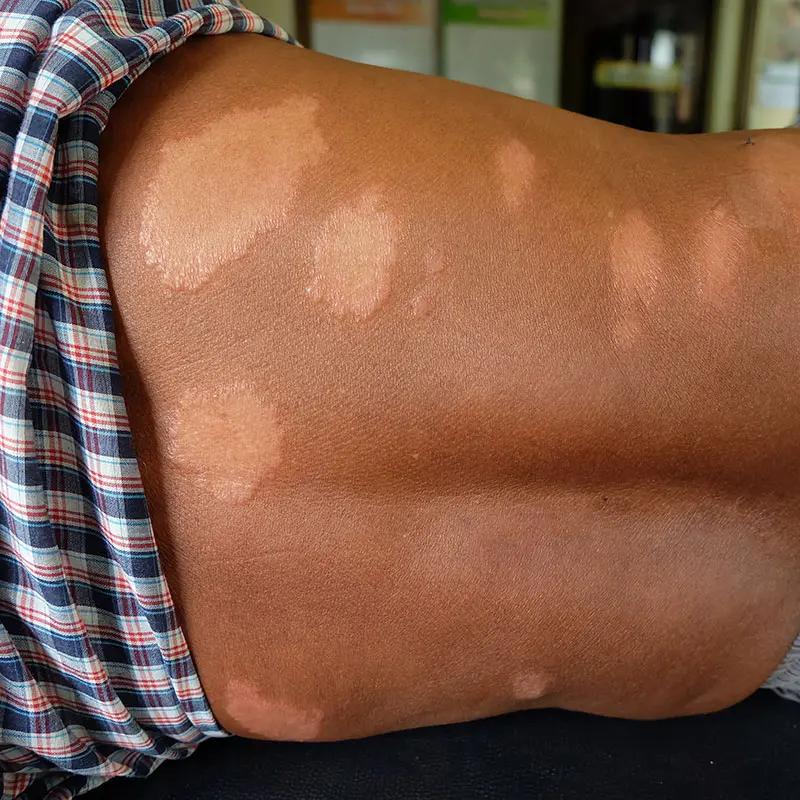
Leprosy
Mycobacterium leprae, the causative agent of leprosy, has been impacting humans for centuries. It differs from other mycobacterial pathogens because it cannot be cultured in vitro from patient specimens.
The available molecular diagnostic methods are unable to distinguish if the M. leprae cells detected are viable or dead, and hence are unable to determine if the patient is infectious, or to assess if chemotherapy has been effective.
Currently, the only available method to demonstrate M. leprae viability remains the mouse footpad assay, which generally takes up to 6 months to deliver results.
The RAPIDvLEP test will work in the same way as the other PhMS-qPCR assays and will definitively detect active M. leprae infection within 24-48 hours rather than ≥ 6 months.
Just as for the other PhMS-qPCR applications the speed, simplicity, sensitivity, and specificity of RAPIDvLEP will transform the routine diagnosis and effective treatment of Leprosy, and has the potential to become the global diagnostic of choice.