About Rapid-myco Technologies
Rapid-myco technologies is developing a truly transformative diagnostic platform for the rapid detection of important and often life-threatening mycobacterial infections of humans and livestock.


Novel, Rapid Phage-based Tests
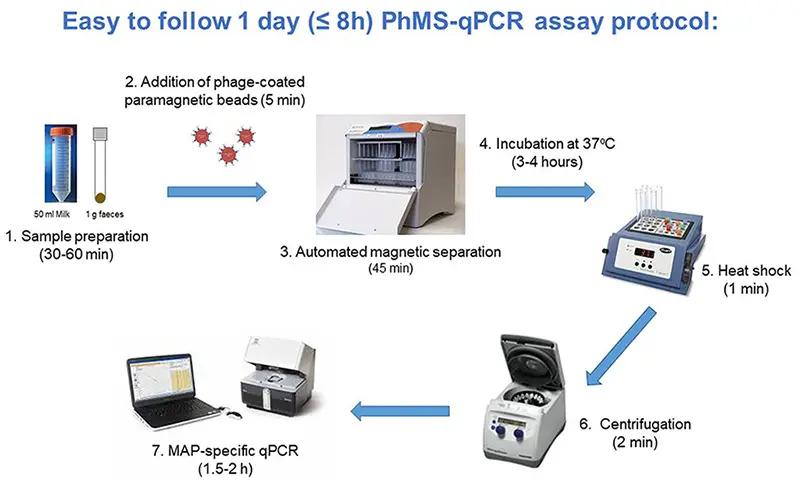
RMT has developed a novel, rapid (≤ 8hrs) bacteriophage (phage)-based qPCR test for viable, ‘active’ Mycobacterium spp.; the only phage-based qPCR test that can work with milk, blood, faecal and, potentially, sputum samples.
The patent-pending technology developed by Prof. Irene Grant at Queen’s University Belfast has the potential to detect many, if not all, of the important mycobacteria which cause sometimes life-threatening infections in humans and ruminant livestock.
The first commercial adaptation of the technology, RAPIDvMAP, is for the rapid detection of Mycobacterium avium subspecies paratuberculosis (MAP), which is the causative agent of Johne’s disease in ruminant livestock.
Other applications of the phage technology platform being developed include tests for bovine TB (bTB), zoonotic bovine TB which affects humans (zTB), human TB (TB) and leprosy.
Livestock Applications
Read more about the applications for Johne’s Disease and Bovine Tuberculosis.
complementary lateral flow test
In addition to its proprietary phage technology, RMT has also developed a complementary lateral flow test (LFT) for TB and bTB.
Uniquely, this screening test will be able to differentiate between M. bovis (zTB) and other M. tuberculosis complex (TB) infected patients in under 15 minutes after traditional sputum mycobacterial culture.
The clinical application of these tests would be as rapid screening tests to be used in developing countries where access to the sophisticated post-culture spoligotyping techniques required to characterise and differentiate mycobacterial species is limited or unavailable.

